Background
Results from the phase 3 QuANTUM-First study (NCT02668653) showed that in patients (pts) with newly diagnosed (nd) acute myeloid leukemia (AML) and positive for FMS-like tyrosine kinase 3-internal tandem duplication ( FLT3-ITD+), the addition of the oral, highly potent, and selective, type 2 FLT3 inhibitor quizartinib (Quiz) to standard chemotherapy ± allogeneic hematopoietic cell transplantation (allo-HCT), followed by continuation monotherapy for up to 3 years, decreased the relative risk of death by 22.4% vs placebo (PBO) (PMID: 37116523). We assessed 1) the impact of CR duration on OS and EFS and 2) the kinetics of CR achievement over time after induction.
Methods
QuANTUM-First enrolled pts aged 18-75 years with FLT3-ITD+ nd AML, randomized to receive standard induction chemotherapy with either Quiz (40 mg/d) or PBO, and stratified by region (Europe, North America, or Asia/Australia/South America), age (<60 years, ≥60 years), and white blood cell count (WBC; <40×10 9/L, ≥40×10 9/L) at diagnosis. Pts who achieved CR or CR with incomplete hematologic recovery (CRi) received up to 4 cycles of high-dose cytarabine combined with Quiz (40 mg/d) or PBO and/or allo-HCT followed by up to 3 years (36 cycles) of continuation therapy with Quiz (30-60 mg/d) or PBO. OS was the primary endpoint, EFS and rates of CR and composite complete remission (CRc) were secondary endpoints; CR duration was an exploratory endpoint. The impact of CR duration on OS was analyzed by extended Cox regression model stratified by region, age, and WBC count at diagnosis and included treatment group and CR duration status (time-dependent covariate). CR duration status was 0 for pts who did not achieve CR, 1 for CR pts throughout the duration of sustained CR, and 0 for CR pts after they relapsed.
Results
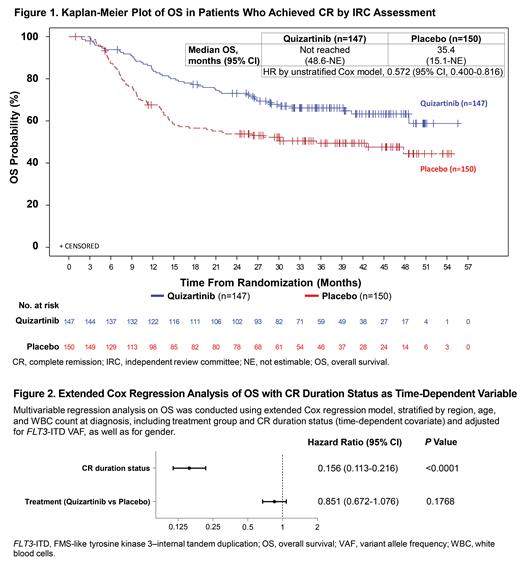
Of the 539 randomized pts enrolled in QuANTUM-First (Quiz, n=268; PBO, n=271), there were 297 CR pts (Quiz, n=147; PBO, n=150) based on independent review committee assessment after 1 to 2 courses of induction. Among 533 treated pts, 20% had 2 induction cycles. Baseline characteristics in CR pts were well balanced between arms: median age in years (range), 56.0 (23-75) vs 55.5 (20-74); females, 55.8% vs 60.0%; ECOG ≥1, 64.6% vs 63.3%; mutated NPM1 / CEBPA,66.7% vs 64.7% / 25.9% vs 23.3%; variant allele frequency (VAF) >25%, 68.7% vs 66.7%; and WBC count at diagnosis ≥40×10 9/L, 55.8% vs 49.3%. Median OS in CR pts was not reached (95% CI 48.6 months-NE) in the Quiz arm vs 35.4 (95% CI 15.1-NE) months in the PBO arm (HR 0.572, 95% CI 0.400-0.816; Figure 1). Although CR rates were similar between arms (55% in both), median CR duration was longer with Quiz vs PBO (38.6 months, 95% CI 21.9-NE vs 12.4 months, 95% CI 8.8-22.7; HR 0.621, 95% CI 0.451-0.857). Based on the extended Cox model, CR duration status covariate was strongly predictive for OS (HR 0.156, 95% CI 0.113-0.216; nominal P<0.0001; Figure 2). Importantly, the HR for Quiz vs PBO effect on OS in the model with the time-dependent covariate CR duration status was higher (HR 0.851, 95% CI 0.672-1.076) vs the HR of the primary OS analysis (HR 0.776, 95% CI 0.615-0.979), implying that a substantial fraction of OS benefit was mediated through its effects on achieving a durable CR (Figure 2). EFS favored Quiz over PBO when induction treatment failure (ITF) was defined as not achieving CRc or CR, by the end of induction up to day 56 from the last induction cycle, with HR 0.729 (95% CI 0.592-0.897; nominal P=0.0031) and 0.818 (95% CI 0.669-0.999; nominal P=0.032), respectively. EFS was similar between arms when ITF was defined, according to FDA guidance, as not achieving CR by day 42 from the start of the last induction cycle (HR 0.916, 95% CI 0.754-1.114; P=0.24; primary EFS analysis). Between day 42 and the end of the induction phase, there were 51 pts who achieved CR (Quiz, n=33; PBO, n=18), and they were considered as ITF with EFS event on day 1 in the primary analysis of EFS. Among these 51 pts, 9 (Quiz, n=5; PBO, n=4) had CRi by day 42 and became CR after day 42 in the induction phase.
Conclusions
These analyses demonstrated that in QuANTUM-First: 1) a substantial fraction of the estimated effect of Quiz on OS was mediated through its effect on achieving a durable CR and 2) more pts in the Quiz arm vs the PBO arm achieved CR after day 42 of the last induction cycle, suggesting that there was a delay in CR achievement with Quiz. Longer EFS was observed in Quiz over PBO based on the EFS definition including CRs between day 42 and end of induction.
Disclosures
Montesinos:GILEAD: Consultancy; BEIGENE: Consultancy; Takeda: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Celgene: Consultancy; NERVIANO: Consultancy; Janssen: Speakers Bureau; Kura oncology: Consultancy; Menarini-Stemline: Consultancy, Research Funding; INCYTE: Consultancy; Astellas: Consultancy, Speakers Bureau; Ryvu: Consultancy; OTSUKA: Consultancy; Jazz pharma: Consultancy, Research Funding, Speakers Bureau; Abbvie: Consultancy, Research Funding, Speakers Bureau; Pfizer: Consultancy, Research Funding, Speakers Bureau; BMS: Consultancy, Other, Research Funding; Daiichi Sankyo: Consultancy, Research Funding. Perl:Syndax: Research Funding; Genentech: Honoraria; BerGen Bio: Honoraria; Abbvie: Consultancy, Honoraria, Research Funding; Astellas: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bayer: Research Funding; FujiFilm: Research Funding; Forma: Consultancy; Foghorn: Consultancy; Beat AML: Other: Participation on a Data Safety Monitoring Board or Advisory Board; Immunogen: Honoraria; BMS: Honoraria; Aptose: Honoraria; Daiichi-Sankyo: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Rigel: Honoraria; Actinium: Honoraria. Sekeres:Geron: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kurome: Consultancy, Current holder of stock options in a privately-held company; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees. Liu:Daiichi Sankyo Inc.: Current Employment. Kamel:Daiichi Sankyo Inc.: Consultancy, Other. Schlenk:Daiichi Sankyo: Consultancy, Honoraria, Other: Steering Committee; Receipt of equipment, materials, drugs, medical writing, gifts or other services; Pfizer: Consultancy, Honoraria, Other: Receipt of equipment, materials, drugs, medical writing, gifts or other services; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Jazz: Consultancy; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Other: Equiptment, materials medical writing, gifts, other services; PharmaMar: Other: equipment, materials medical writing, gifts, other services; AstraZeneca: Other: Receipt of equipment, materials, drugs, medical writing, gifts or other services; Boehringer Ingelheim: Other: Receipt of equipment, materials, drugs, medical writing, gifts or other services. Erba:Kura Oncology: Consultancy, Research Funding; Trillium: Consultancy; Forma: Research Funding; ALX Oncology: Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Amgen: Research Funding; Servier: Consultancy, Honoraria, Research Funding; Macrogenics: Consultancy, Research Funding; Syros: Consultancy; Pfizer: Consultancy; Forty-Seven: Research Funding; Gilead: Research Funding; Sunesis Pharmaceuticals: Honoraria; Takeda: Consultancy; Ascentage: Research Funding; Jazz Pharma: Consultancy, Honoraria, Research Funding; Incyte: Consultancy, Honoraria; Daiichi Sankyo Inc.: Consultancy, Research Funding; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Glycomimetics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Consultancy; Agios: Consultancy, Honoraria, Research Funding; Astellas: Consultancy; BMS: Consultancy, Honoraria, Other: Chair, Myeloid Neoplasms Repository Study; Celgene: Consultancy, Honoraria, Other: Chair, Myeloid Neoplasms Repository Study, Research Funding; Immunogen: Consultancy, Research Funding; PTE: Research Funding; Sumitomo: Research Funding. Dombret:Pfizer: Research Funding; Jazz Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Support for attending meetings; Astellas: Research Funding; Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.